Difference between revisions of "Projects:QuantitativeSusceptibilityMapping"
| Line 86: | Line 86: | ||
subjects. Obtaining FDRI measurements would be impractical for most studies, | subjects. Obtaining FDRI measurements would be impractical for most studies, | ||
however, since it requires images to be collected on two separate scanners. | however, since it requires images to be collected on two separate scanners. | ||
| − | + | In this work, quantitative results are obtained by comparison of mean susceptibility | |
| + | values in the thalamus (TH), caudate (CD), putamen (PT) | ||
and globus pallidus (GP) to corresponding results from SWI, FDRI and postmortem data. | and globus pallidus (GP) to corresponding results from SWI, FDRI and postmortem data. | ||
Revision as of 23:06, 24 March 2011
Home < Projects:QuantitativeSusceptibilityMappingQuantifying magnetic susceptibility in the brain from the phase of the MR signal provides a non-invasive means for measuring the accumulation of iron believed to occur with aging and neurodegenerative disease. Phase observations from local susceptibility distributions, however, are corrupted by external biasfields, which may be identical to the sources of interest. Furthermore, limited observations of the phase makes the inversion ill-posed. We describe a variational approach to susceptibility estimation that incorporates a tissue-air atlas to resolve ambiguity in the forward model, while eliminating additional biasfields through application of the Laplacian. Results show qualitative improvement over two methods commonly used to infer underlying susceptibility values, and quantitative susceptibility estimates show better correlation with postmortem iron concentrations than competing methods.
Description
There is increasing evidence that excessive iron deposition in specific regions of the brain is associated with neurodegenerative disorders such as Alzheimer's and Parkinson's disease [1]. The role of iron in the pathogenesis of these diseases remains unknown and is difficult to determine without a non-invasive method to quantify its concentration in-vivo. Since iron is a ferromagnetic substance, changes in iron concentration result in local changes in the magnetic susceptibility of tissue. In magnetic resonance imaging (MRI) experiments, differences in magnetic susceptibility cause perturbations in the local magnetic field, which can be computed from the phase of the MR signal (in a gradient echo sequence, the observed field is proportional to the MR phase).
The field perturbations caused by magnetic susceptibility differences can be
modeled as the convolution of a dipole-like kernel with the spatial susceptibility
distribution. In the Fourier domain, the kernel exhibits zeros at the magic angle,
preventing direct inversion of the fieldmap [3]. Critically, limited observations of
the field make the problem ill-posed. The observed data is also corrupted by
confounding biasfields (ie. those from tissue-air interfaces, mis-set shims, and other
non-local sources). Eliminating these fields is critical for accurate susceptibility
estimation since they corrupt the phase contributions from local susceptibility
sources.
In general, methods that rely heavily on agreement between observed and
predicted field values computed using kernel-based forward models [2, 3, 6] are
inherently limited since they cannot distinguish between low frequency biasfields
and susceptibility distributions that are eigenfunctions of the model. Examples
of such distributions include constant, linear, and quadratic functions of
susceptibility along the main field (ie. 'z') direction. Applying the forward model to
these distributions results in predicted fields that are proportional to the local
susceptibility sources, but also identical in form to non-local biasfields (ie. those
produced by a z-shim). Therefore, removing all low frequency fields prior to
susceptibility estimation will eliminate the biasfield as well as fields due to the
sources of interest, potentially preventing accurate calculation of the underlying
susceptibility values. In contrast, inadequate removal of the biasfield may result
in the estimation of artifactual susceptibility eigenfunctions in areas where the
biasfield is strong, such as regions adjacent to tissue-air interfaces. This suggests
that additional information such as boundary conditions or priors may be necessary to
regularize an incomplete forward model and prevent the mis-estimation
of low frequency biasfields.
We present a variational approach for Atlas-based Susceptibility Mapping
(ASM) that performs simultaneous susceptibility estimation and biasfield
removal using the Laplacian operator and a tissue-air susceptibility atlas. In [7,
8, 6] it was shown that applying the Laplacian to the observed field eliminates
non-local biasfields due to mis-set shims and remote susceptibility
distributions (ie. the neck/chest).
In this method, large deviations from the susceptibility atlas are penalized,
discouraging the estimation of artifactual susceptibility eigenfunctions in regions near
tissue-air boundaries where the Laplacian may not be sufficient to eliminate the
contribution of non-local sources and substantial signal loss corrupts the observed field.
Agreement of predicted and observed fields
within the brain is also enforced, but deviations in estimated susceptibility values outside the
brain are not penalized, allowing values at the boundary to vary from
the atlas-based prior to account for unmodeled external field sources (ie. shims).
Results
The method is evaluated by comparison of susceptibility maps estimated using ASM to results from Susceptibility Weighted Imaging (SWI) and Field Dependent Relaxation Imaging (FDRI). In SWI, a filtered phase map is obtained by applying a high-pass filter to the phase data, and the resulting SWI map is commonly used as a proxy for susceptibility. While SWI has shown some correlation with magnetic susceptibility differences due to iron and other sources, the phase maps it yields are only an indirect measure of susceptibility due to the non-local effects of the convolution kernel. In addition, the filtering process may remove some low frequency fields due to sources inside the brain. In FDRI, R2 maps are acquired at two different field strengths (ie. 1.5 and 3 Tesla) and the difference in R2 divided by the difference in field strength gives the FDRI. The mean FDRI in several regions of interest was previously compared to the mean iron concentration obtained from postmortem analysis and showed stronger correlation with iron content than the SWI maps computed for the same subjects. Obtaining FDRI measurements would be impractical for most studies, however, since it requires images to be collected on two separate scanners. In this work, quantitative results are obtained by comparison of mean susceptibility values in the thalamus (TH), caudate (CD), putamen (PT) and globus pallidus (GP) to corresponding results from SWI, FDRI and postmortem data.
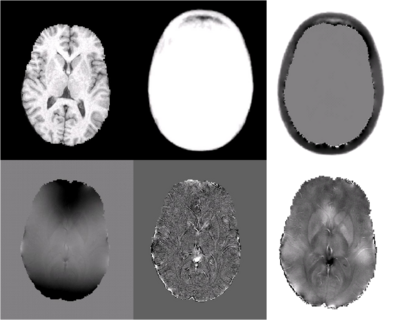
ASM results for a young subject are shown in Fig. 1. Column 1 shows the T1
structural (row 1) and acquired fieldmap (row 2). Application of the Laplacian
to the field map (row 2, column 2) removes substantial B0 inhomogeneities that
bias the observed field. The susceptibility atlas is shown in row 1, column 2
and estimated external sources are shown in row 1, column 3. The estimated
susceptibility map (row2, column 3) shares high frequency structure with the
Laplacian of the observed field, while low frequency structure is preserved by
enforcing agreement with additional information provided by the atlas-based
prior and observed field.
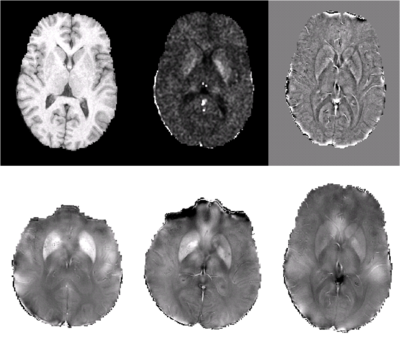
Fig. 2 shows results from FDRI (row 1, column 2), SWI (row 1,column 3), ASM for a young subject (row 2, column 3), and ASM results for 2 elderly subjects (row 2, columns 1,2). The FDRI shows strong constrast between the ROIs and surrounding tissue, but less high frequency structure than the SWI. The SWI retains high frequency phase effects, but indiscriminately removes low order fields from both internal and external sources, resulting in artifactual low frequency structure. The ASM method accurately preserves the high frequency phase effects seen in SWI while showing improved estimation of low order susceptibility distributions. In addition, ASM provides direct estimates of susceptibility values rather than filtered phase proxies for susceptibility.
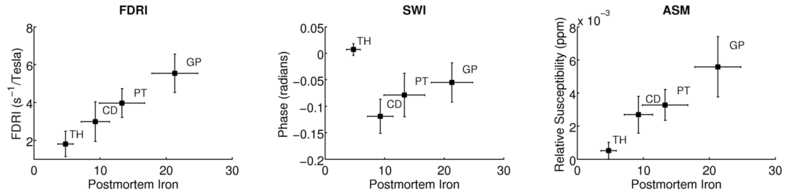
Quantitative results from ASM and previously reported results from FDRI
and SWI for the same elderly subjects are shown in Fig. 3. The mean
susceptibility values (relative to tissue susceptibility) in each ROI from all elderly subjects are plotted
against the corresponding iron concentrations from postmortem analysis (only
the mean and SD in each ROI was reported in [17]). ASM shows a high
correlation with postmortem values, which is comparable to that seen in FDRI and
substantially better than the correlation between phase and iron concentration
obtained with SWI. In addition, for the structures that we analyzed, ASM results
compare favorably to the correlation between postmortem iron and
susceptibility estimates in corresponding ROIs computed from multi-angle acquisitions [6].
Key Investigators
- MIT: Clare Poynton, Elfar Adalsteinsson, Polina Golland
- BWH/Harvard: William Wells
- Stanford: Adolf Pfefferbaum, Edith Sullivan