Difference between revisions of "2017 Winter Project Week/3DSurgicalPlanningBreastReconstruction"
| (10 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | Key Investigators | + | __NOTOC__ |
| + | <gallery> | ||
| + | Image:PW-Winter2017.png|link=2017_Winter_Project_Week#Projects|[[2017_Winter_Project_Week#Projects|Projects List]] | ||
| + | <!-- Use the "Upload file" link on the left and then add a line to this list like "File:MyAlgorithmScreenshot.png" --> | ||
| + | Image:Chae2015-Fig4.png | ||
| + | File:5.JPG | ||
| + | File:6.JPG | ||
| + | </gallery> | ||
| + | |||
| + | ==Key Investigators== | ||
* Michael Chae (Monash University, Australia) | * Michael Chae (Monash University, Australia) | ||
| − | * Andras Lasso (Queen’s University, Canada) | + | * Andras Lasso (Queen’s University, Canada) |
| + | * David Garcia (Queen’s University, Canada) | ||
* Julian Smith (Monash University, Australia) | * Julian Smith (Monash University, Australia) | ||
* Warren Rozen (Monash University, Australia) | * Warren Rozen (Monash University, Australia) | ||
* David Hunter-Smith (Monash University, Australia) | * David Hunter-Smith (Monash University, Australia) | ||
| − | Project | + | ==Project Description== |
| + | {| class="wikitable" | ||
| + | ! style="text-align: left; width:27%" | Objective | ||
| + | ! style="text-align: left; width:27%" | Approach and Plan | ||
| + | ! style="text-align: left; width:27%" | Progress and Next Steps | ||
| + | |- style="vertical-align:top;" | ||
| + | | | ||
| + | <!-- Objective bullet points --> | ||
| + | |||
| + | * Develop tools to help surgeons plan breast reconstructive surgeries in patients with breast cancer: | ||
| + | ** Volumetric analysis based on surface scans and pre-op volumetric images | ||
| + | ** 3D-printed surgical planning template | ||
| + | * Current method requires extensive manual handling and is subsequently slow. We are aiming to develop automated or semi-automated techniques. | ||
| + | * Implement interface for inexpensive 3D scanners (Intel RealSense cameras) in Slicer | ||
| + | |||
| + | | | ||
| + | <!-- Approach and Plan bullet points --> | ||
| + | |||
| + | * Volumetric analysis: | ||
| + | ** Current method [Chae2014]: We can perform volumetric analysis on any imaging platforms (e.g. CT, MRI, 3D scanners). For CT/MRI scanners, we’d manually segment “areas of interest” (i.e. total breast tissue, mammary tissue, breast implants) on their axial slices in Osirix software. We’d refer to 3D-reconstructed image of the breasts (on 3D Slicer) to help guide areas that we’d need to segment. We’re increasing finding it easier to segment certain areas (i.e. mammary tissue, breast implants) from axial slices loaded on 3D slicer, instead of Osirix. For 3D Scanner-derived images, we’d upload the 3D file on to MeshMixer software, from which the file will be meshed (e.g. cutting, making planes). These files will be sent to Blender software for volume calculation. | ||
| + | ** We have been collecting the thresholding values used for segmenting breast tissues. We’d like to use these metrics, or other means, to automate/semi-automate breast volumetric analysis techniques | ||
| + | |||
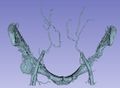
| + | * 3D printed surgical planning template: In autologous breast reconstruction, we raise a flap of tissue (i.e. abdominal wall fat) based on a perforator vessel called, DIEP (deep inferior epigastric artery perforators) perforators. These course through muscle called rectus abdominis (aka “six packs”). We’d like to 3D print the patient-specific DIEP perforators and their surrounding rectus abdominis muscle for surgical planning. Currently, we can achieve this on 3D Slicer (manual segmentation via watershed method, island effect tool, and thresholding) but this is very difficult and time-consuming. It’ll be a great opportunity to make this process easier and also automate/semi-automate it. | ||
| + | |||
| + | | | ||
| + | <!-- Progress and Next steps bullet points (fill out at the end of project week --> | ||
| + | |||
| + | Outcomes of our project from this year’s project week is outlined below: | ||
| − | |||
| − | |||
# Volumetric analysis | # Volumetric analysis | ||
| − | # 3D | + | ## Purchase Intel RealSense 3D scanner at Monash University and use it for planning breast reconstructions |
| − | However, | + | ## Image patient’s arms in Akimbo |
| + | ## Create breast’s posterior plane by identifying breast margins using fiducials | ||
| + | ### However, there is a room for investigation here to see if an artificial “flat” posterior plane is going to be more clinically useful since it accounts for potential asymmetry in the chest wall (i.e. ribs, pectoralis muscles) | ||
| + | ## Derive volume using “Segment Statistics” module | ||
| + | ## Apply into case series for publication | ||
| + | |||
| + | # 3D printed surgical planning template | ||
| + | ## The new Segmentation Editor module has streamlined everything | ||
| + | ## What we need to segment is the DIEA (deep inferior epigastric artery) from its origin (i.e. common femoral artery), DIEP (deep inferior epigastric artery perforator), rectus abdominis muscle, and overlying skin | ||
| + | ## Segmenting skin: threshold, regiongrowing by ~5 mm, subtract | ||
| + | ## Segmenting muscle: threshold paint, draw out the muscle at various points on axials, automatically fill in the gaps | ||
| + | ## Segmenting DIEA and DIEP: VMTK, region growing, still work to do to streamline this process | ||
| + | |||
| + | * Current sources of funding and collaborative efforts elsewhere: | ||
| + | ** MIME (Monash Institute of Medical Engineering) Seedfund grant (for 3D bioprinting), Monash University (Funding ID M17001/3167528) | ||
| + | ** Development of a prototype volumetric analysis tool for CT and MR guided 3D printing | ||
| + | ** Development of a 3D printed templating technique for vascular mapping in reconstructive surgery | ||
| + | ** Founding of The Peninsula 3D Printing Laboratory, for surgical 3D printing clinical and research applications. | ||
| + | ** Collaborative 3D printing research projects with: | ||
| + | *** Monash Institute of Medical Engineering (MIME), Materials Engineering Department, Monash University - "3D-bioprinted scaffold of trapezium in basal thumb arthritis management". | ||
| + | *** Hudson Institute of Medical Research, Monash University – “3D Bioprinting in Reconstructive Surgery”. | ||
| + | *** St Andrew’s Centre for Plastic and Reconstructive Surgery, Broomfield Hospital, UK – “Volumetric and Imaging Analysis in Breast Reconstruction”. | ||
| − | |||
| − | + | |} | |
| − | Background and | + | ==Background and References== |
| + | <!-- Use this space for information that may help people better understand your project, like links to papers, source code, or data --> | ||
1 in 8 women in the US will be diagnosed with breast cancer in their lifetime. As genetic testing for breast cancer, such as BRCA1/2, becomes more available, an increasing number of women will be diagnosed early and evidences show that more and more women are opting for aggressive surgery (i.e. mastectomy) early on to achieve cure. As a result, post-mastectomy breast reconstruction has become an important component of the holistic treatment of patients with breast cancer. Breast reconstruction with autologous tissue (i.e. one’s own tissue) bypasses risks associated with traditional implants and provides a stable, natural-appearing, long-term volume replacement. The most ideal source of tissue for breast reconstruction is the abdominal wall. These tissues are raised as a free flap tissue based on small vessels, called perforators. Unfortunately, there is a significant variance in perforator size and locations between individuals. Advancements in modern imaging technologies, such as computed tomographic angiography (CTA), has enabled surgeons to select the appropriate perforator and facilitate flap design, leading to improvements in clinical outcomes. However, their efficacy is limited by being displayed on a two-dimensional (2D) surface. In contrast, imaging-guided 3D-printed surgical planning solution can provide tactile feedback and a superior appreciation of visuospatial relationship between anatomical structures. (1-4) | 1 in 8 women in the US will be diagnosed with breast cancer in their lifetime. As genetic testing for breast cancer, such as BRCA1/2, becomes more available, an increasing number of women will be diagnosed early and evidences show that more and more women are opting for aggressive surgery (i.e. mastectomy) early on to achieve cure. As a result, post-mastectomy breast reconstruction has become an important component of the holistic treatment of patients with breast cancer. Breast reconstruction with autologous tissue (i.e. one’s own tissue) bypasses risks associated with traditional implants and provides a stable, natural-appearing, long-term volume replacement. The most ideal source of tissue for breast reconstruction is the abdominal wall. These tissues are raised as a free flap tissue based on small vessels, called perforators. Unfortunately, there is a significant variance in perforator size and locations between individuals. Advancements in modern imaging technologies, such as computed tomographic angiography (CTA), has enabled surgeons to select the appropriate perforator and facilitate flap design, leading to improvements in clinical outcomes. However, their efficacy is limited by being displayed on a two-dimensional (2D) surface. In contrast, imaging-guided 3D-printed surgical planning solution can provide tactile feedback and a superior appreciation of visuospatial relationship between anatomical structures. (1-4) | ||
| − | + | * Chae, M. P., Hunter-Smith, D. J., Spychal, R. T., Rozen, W. M. 3D volumetric analysis for planning breast reconstructive surgery. Breast Cancer Res Treat 2014;146:457-460. | |
| − | + | * Rozen, W. M., Phillips, T. J., Ashton, M. W., Stella, D. L., Gibson, R. N., Taylor, G. I. Preoperative imaging for DIEA perforator flaps: a comparative study of computed tomographic angiography and Doppler ultrasound. Plast Reconstr Surg 2008;121:9-16. | |
| − | + | * Masia, J., Clavero, J. A., Larranaga, J. R., Alomar, X., Pons, G., Serret, P. Multidetector-row computed tomography in the planning of abdominal perforator flaps. J Plast Reconstr Aesthet Surg 2006;59:594-599. | |
| − | + | * Chae, M. P., Rozen, W. M., McMenamin, P. G., Findlay, M. W., Spychal, R. T., Hunter-Smith, D. J. Emerging Applications of Bedside 3D Printing in Plastic Surgery. Front Surg 2015;2:25. | |
| + | * Gerstle, T. L., Ibrahim, A. M., Kim, P. S., Lee, B. T., Lin, S. J. A plastic surgery application in evolution: three-dimensional printing. Plast Reconstr Surg 2014;133:446-451. | ||
Latest revision as of 07:12, 13 January 2017
Home < 2017 Winter Project Week < 3DSurgicalPlanningBreastReconstructionKey Investigators
- Michael Chae (Monash University, Australia)
- Andras Lasso (Queen’s University, Canada)
- David Garcia (Queen’s University, Canada)
- Julian Smith (Monash University, Australia)
- Warren Rozen (Monash University, Australia)
- David Hunter-Smith (Monash University, Australia)
Project Description
| Objective | Approach and Plan | Progress and Next Steps |
|---|---|---|
|
|
Outcomes of our project from this year’s project week is outlined below:
|
Background and References
1 in 8 women in the US will be diagnosed with breast cancer in their lifetime. As genetic testing for breast cancer, such as BRCA1/2, becomes more available, an increasing number of women will be diagnosed early and evidences show that more and more women are opting for aggressive surgery (i.e. mastectomy) early on to achieve cure. As a result, post-mastectomy breast reconstruction has become an important component of the holistic treatment of patients with breast cancer. Breast reconstruction with autologous tissue (i.e. one’s own tissue) bypasses risks associated with traditional implants and provides a stable, natural-appearing, long-term volume replacement. The most ideal source of tissue for breast reconstruction is the abdominal wall. These tissues are raised as a free flap tissue based on small vessels, called perforators. Unfortunately, there is a significant variance in perforator size and locations between individuals. Advancements in modern imaging technologies, such as computed tomographic angiography (CTA), has enabled surgeons to select the appropriate perforator and facilitate flap design, leading to improvements in clinical outcomes. However, their efficacy is limited by being displayed on a two-dimensional (2D) surface. In contrast, imaging-guided 3D-printed surgical planning solution can provide tactile feedback and a superior appreciation of visuospatial relationship between anatomical structures. (1-4)
- Chae, M. P., Hunter-Smith, D. J., Spychal, R. T., Rozen, W. M. 3D volumetric analysis for planning breast reconstructive surgery. Breast Cancer Res Treat 2014;146:457-460.
- Rozen, W. M., Phillips, T. J., Ashton, M. W., Stella, D. L., Gibson, R. N., Taylor, G. I. Preoperative imaging for DIEA perforator flaps: a comparative study of computed tomographic angiography and Doppler ultrasound. Plast Reconstr Surg 2008;121:9-16.
- Masia, J., Clavero, J. A., Larranaga, J. R., Alomar, X., Pons, G., Serret, P. Multidetector-row computed tomography in the planning of abdominal perforator flaps. J Plast Reconstr Aesthet Surg 2006;59:594-599.
- Chae, M. P., Rozen, W. M., McMenamin, P. G., Findlay, M. W., Spychal, R. T., Hunter-Smith, D. J. Emerging Applications of Bedside 3D Printing in Plastic Surgery. Front Surg 2015;2:25.
- Gerstle, T. L., Ibrahim, A. M., Kim, P. S., Lee, B. T., Lin, S. J. A plastic surgery application in evolution: three-dimensional printing. Plast Reconstr Surg 2014;133:446-451.