Difference between revisions of "AHM2010:Mind"
Hjbockholt (talk | contribs) m (→Team) |
Hjbockholt (talk | contribs) m |
||
| Line 60: | Line 60: | ||
** Scully, M., Anderson, B., Lane, T., Gasparovic, C., Magnotta, V., Sibbitt, W., Roldan,C. , Kikinis, R., Bockholt, H. J. An Automated Method For Longitudinal Analysis of White Matter Lesions in Lupus (in preparation for submission). | ** Scully, M., Anderson, B., Lane, T., Gasparovic, C., Magnotta, V., Sibbitt, W., Roldan,C. , Kikinis, R., Bockholt, H. J. An Automated Method For Longitudinal Analysis of White Matter Lesions in Lupus (in preparation for submission). | ||
** Bockholt, H.J., Gasparovic, C., Scully, M., Magnotta, V., Sibbitt, W., Kikinis, R., Roldan,C. A novel white matter lesion analysis for improved clinical application in lupus. (in preparation for submission). | ** Bockholt, H.J., Gasparovic, C., Scully, M., Magnotta, V., Sibbitt, W., Kikinis, R., Roldan,C. A novel white matter lesion analysis for improved clinical application in lupus. (in preparation for submission). | ||
| + | |||
| + | ==Progress since last AHM== | ||
| + | *Longitudinal Analyses | ||
| + | filling this in still | ||
| + | *DTI Analyses | ||
| + | filling this in still | ||
| + | *Multiscale Analyses | ||
| + | filling this in still | ||
==Future Collaboration== | ==Future Collaboration== | ||
Revision as of 18:13, 6 January 2010
Home < AHM2010:MindMRN Roadmap Project
Overview
- What problem does the pipeline solve, and who is the targeted user?
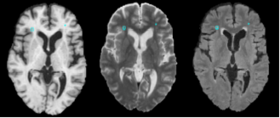
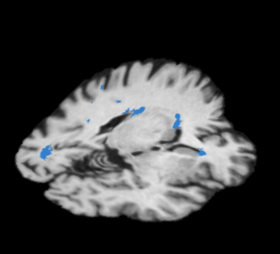
- The pipeline attempts to solve the problem of segmenting white matter lesions in Neuropsychiatric Systemic Lupus Erythematosus(NPSLE). The automated capability is aimed at clinical researchers using Slicer3 software. The utility of having accurate lesion labels permits summary of perfusion within lesions, correlation of lesion load by location with neuropsychiatric symptoms, and summary of structural image intensities or DTI scalars within lesion boundaries.
- How does the pipeline compare to state of the art?
- The state of the art in brain lesion classification is a work in progress in NPSLE, as well as, other disorders. There are many approaches that attempt to solve lesion classification in Multiple Sclerosis (where it is much more common to perform neuroimaging studies). Some of these approaches are automated or semi-automated; however, all automatic approaches suffer a lack of ground truth. It is difficult for human manual raters to agree on fuzzy boundaries across different image contrasts (e.g., T1, T2, FLAIR).
A good example of the challenge that remains in terms of lesion segmentation was well represented at the 2008 MICCAI Conference MS Lesion Segmentation Challenge(organized by Warfield and Styner) this past September in the form of a grand challenge in segmentation. Several groups, including this DBP, competed in lesion segmentation contest, participants were first provided training data-sets with two manual labellings, their respective approaches were judged in an on-site, real-time competition on additional novel data sets.
Through participation in the recent lesion challenge, a thorough literature review of recent advances, and trying out several approaches (EM-Segment(S. Wells), k-means/bayesian(V. Magnotta), outlier detection(M. Prastawa), etc.), we think we have honed in on a novel methodology that will perform well for providing lesion maps in NPSLE; in addition, generalize well (following training) for other neurological disorders, such as MS, vascular dementia, and other disorders of the brain.
Detailed Information about the Pipeline
- Illustrate the components and workflow of the pipeline using your own data
- Method
- A synergistic combination of supervised machine learning methods
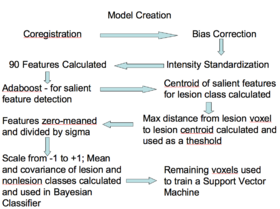
- Training
- Approximately 90 features were computed based on the T1, T2, and FLAIR, including neighborhood means with varying radii, mathematical morphometry dilation and erosion, kmeans clustering, and gradients, among others
- Adaboost was applied to the data to find the 20 features that best discriminate lesion from non-lesion
- Those 20 features were then calculated for all lesions, for all subjects, then zero-meaned and the standard deviation was set to one.
- The centroid of the lesions was then calculated and the max distance found between the centroid and the lesion voxels
- The max distance threshold was used to exclude voxels that had no chance of being lesions
- The features for all voxels within the distance threshold were calculated and scaled to a range of negative one to positive one
- The means and covariance of these features were calculated for both the lesion and non-lesion classes and used to define the two classes in a Bayesian classifier
- A parameter search was then performed to find the prior that gave the best combination of Specificity and Sensitivity.
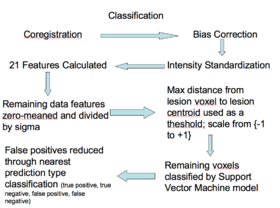
- Classifying
- The 20 relevant features are calculated, zero-meaned and sigma set to one, thresholded based on the distance to the lesion centroid, and then passed to the Bayesian classifier
- Method
Software & documentation
- We have a very active project for this pipeline on the NITRC resource 3DSlicerLupusLesionModule
Team
- DBP: H Jeremy Bockholt (PI), Charles Gasparovic(co-PI), Mark Scully(Engineer), The Mind Research Network
- Core 1: Ross Whitaker, University of Utah
- Core 2: Steve Pieper, Isomics
- Consultant: Vincent Magnotta, University of Iowa
- Contact: H. Jeremy Bockholt, jbockholt@mrn.org
Outreach
- An end-to-end tutorial and module are provided on the NA-MIC website. Training courses were held at the Annual meeting for Human Brain Mapping Organization 2008, the Annual Meeting for Society for Neuroscience 2008, as well as to a group of 25+ investigators at the MIND Research Network (2007) who are working in translational neuroscience. A Manuscript summarizing improved clinical results resulting from automated lesion analyses has been prepared and submitted at the time of writing this report.
- Publication Links to the PubDB.
- H. J. Bockholt, V. A. Magnotta, M. Scully, C. Gasparovic, B. Davis, K. Pohl, R. Whitaker, S. Pieper, C. Roldan, R. Jung, R. Hayek, W. Sibbitt, J. Sharrar, P. Pellegrino, R. Kikinis. A novel automated method for classification of white matter lesions in systemic lupus erythematosus. Presented at the 38th annual meeting of the Society for Neuroscience, Washingto, DC, 15 – 19 November 2008
- Scully M., Magnotta V., Gasparovic C., Pelligrimo P., Feis D., Bockholt H.J. 3D Segmentation In The Clinic: A Grand Challenge II at MICCAI 2008 - MS Lesion Segmentation. IJ - 2008 MICCAI Workshop - MS Lesion Segmentation. Available http://grand-challenge2008.bigr.nl/proceedings/pdfs/msls08/282_Scully.pdf
- H Jeremy Bockholt, Josef Ling, Mark Scully, Adam Scott, Susan Lane, Vincent Magnotta, Tonya White, Kelvin Lim, Randy Gollub, Vince Calhoun. Real-time Web-scale Image Annotation for Semantic-based Retrieval of Neuropsychiatric Research Images. Presented at the 14th Annual Meeting of the Organization for Human Brain Mapping, Melbourne, Australia, 15 – 19 June, 2008.
- H Jeremy Bockholt, Sumner Williams, Mark Scully, Vincent Magnotta, Randy Gollub, John Lauriello, Kelvin Lim, Tonya White, Rex Jung, Charles Schulz, Nancy Andreasen, Vince Calhoun. The MIND Clinical Imaging Consortium as an application for novel comprehensive quality assurance procedures in a multi-site heterogeneous clinical research study. Presented at the 14th Annual Meeting of the Organization for Human Brain Mapping, Melbourne, Australia, 15 – 19 June, 2008.
- Bockholt HJ, Scully M, Courtney W, Rachakonda S, Scott A, Caprihan A, Fries J, Kalyanam R, Segall J, de la Garza R, Lane S and Calhoun VD (2009) Mining the mind research network: a novel framework for exploring large scale, heterogeneous translational neuroscience research data sources. Front. Neuroinform. 3:36. doi:10.3389/neuro.11.036.2009
- Scully, M., Anderson, B., Lane, T., Gasparovic, C., Magnotta, V., Sibbitt, W., Roldan,C. , Kikinis, R., Bockholt, H. J. An Automated Method For Segmenting White Matter Lesions in Lupus Through Multi-level Morphometric Feature Classification (submitted to Frontiers in Neuroscience 11/2009).
- Scully, M., Anderson, B., Lane, T., Gasparovic, C., Magnotta, V., Sibbitt, W., Roldan,C. , Kikinis, R., Bockholt, H. J. An Automated Method For Longitudinal Analysis of White Matter Lesions in Lupus (in preparation for submission).
- Bockholt, H.J., Gasparovic, C., Scully, M., Magnotta, V., Sibbitt, W., Kikinis, R., Roldan,C. A novel white matter lesion analysis for improved clinical application in lupus. (in preparation for submission).
Progress since last AHM
- Longitudinal Analyses
filling this in still
- DTI Analyses
filling this in still
- Multiscale Analyses
filling this in still
Future Collaboration
Two grants are under preparation to continue this collaboration. The first grant in progress with a planned submission of Feb 5, 2010 will use the Collaborations with NCBC R01 mechanism. The grant will be titled, “Enhancing Brain Lesion Segmentation in Neurological Disorders.” The aim of this project will be to extend the tools developed in the lupus DBP to other vascular disorders, multiple sclerosis, vascular dementia and other disorders with brain lesions. The grant will be a natural extension of work in the lupus DBP and fits in well with the mission of the NA-MIC by extending tools, analyses and approaches across multiple diseases and challenges.
The second grant will utilize the GPL-style license found within the NA-MIC to explore the commercialization potential of the lesion analysis toolkit. A grant entitled “Novel White Matter Lesion Application” has a planned submission of April 5, 2010 and will use the Neurotechnology Research, Development, and Enhancement SBIR mechanism. This grant will be submitted by PI Bockholt who has recently founded and formed Advanced Biomedical Informatics Group, LLC, an independent for-profit company based in Iowa City, IA. This project will also extend the lupus DBP work through further development of turnkey solution that can be placed directly in the hands of clinicians for evaluating white matter lesions in their lupus patients
References
- Adaboost and Support Vector Machines for White Matter Lesion Segmentation in MR Images. Quddus A, Fieguth P, Basir O. PAMI Lab, Department of Electrical and Computer Engineering, University of Waterloo, Waterloo, ON, Canada. Conf Proc IEEE Eng Med Biol Soc. 2005;1:463-6. http://www.ncbi.nlm.nih.gov/pubmed/17282216
- Architecture for an Artificial Immune System Steven A. Hofmeyr, Stephanie Forrest Evolutionary Computation Winter 2000, Vol. 8, No. 4, Pages 443-473 http://www.mitpressjournals.org/doi/abs/10.1162/106365600568257
- Automated segmentation of white matter lesions in 3D brain MR images, using multivariate pattern classification Zhiqiang Lao; Dinggang Shen; Jawad, A.; Karacali, B.; Dengfeng Liu; Melhem, E.R.; Bryan, R.N.; Davatzikos, C. Biomedical Imaging: Nano to Macro, 2006. 3rd IEEE International Symposium on Volume , Issue , 6-9 April 2006 Page(s): 307 - 310 http://ieeexplore.ieee.org/Xplore/login.jsp?url=/iel5/10818/34114/01624914.pdf?temp=x
- Automatic Segmentation of MS Lesions Using a Contextual Model for the MICCAI Grand Challenge Jonathan H. Morra, Zhuowen Tu, Arthur W. Toga, Paul M. Thompson IJ - 2008 MICCAI Workshop - MS Lesion Segmentation http://www.midasjournal.org/browse/publication/280