Difference between revisions of "DBP2:QueensFinal:2010"
Medcomparts (talk | contribs) |
m (Text replacement - "http://www.slicer.org/slicerWiki/index.php/" to "https://www.slicer.org/wiki/") |
||
| Line 4: | Line 4: | ||
=Overview= | =Overview= | ||
| − | A 3D Slicer based end-to-end application was developed for MRI-guided prostate biopsy (implemented as a [http://www.slicer.org 3D Slicer 3.6] module, [ | + | A 3D Slicer based end-to-end application was developed for MRI-guided prostate biopsy (implemented as a [http://www.slicer.org 3D Slicer 3.6] module, [https://www.slicer.org/wiki/Modules:ProstateNav-Documentation-3.6 ProstateNav]). The application supports multiple targeting devices (transrectal robot [[{{fullurl:{{FULLPAGENAME}}}}#ref_5 5], [{{fullurl:{{FULLPAGENAME}}}}#ref_15 15]], transperineal template, and transperineal robot [[{{fullurl:{{FULLPAGENAME}}}}#ref_17 17], [{{fullurl:{{FULLPAGENAME}}}}#ref_18 18]]) and multiple needle types (for biopsy and seed placement). Automatic and semi-automatic registration of the targeting device to the planning image coordinate system was implemented using 4 line fiducials or 3 orthogonal Z-shaped fiducials [[{{fullurl:{{FULLPAGENAME}}}}#ref_15 15], [{{fullurl:{{FULLPAGENAME}}}}#ref_16 16], [{{fullurl:{{FULLPAGENAME}}}}#ref_18 18]]. During the planning phase, the clinician defines several point targets for biopsy and seed placement. The 3D Slicer platform provides flexible visualization of several diagnostic image types (T2, contrast-enhanced, etc.). In the targeting phase, the software computes targeting parameters for each site that permit the targeting devices to accurately position the needles. The software offers a means to quantitatively assess the accuracy of needle placement. Patient motion during the intervention (dislocation and deformation between the planning and verification images) can be easily visualized, thereby reducing the chance of incorrect needle placement during lengthy procedures. The software is being tested in phantom experiments at three clinical sites: Brigham and Women's Hospital, NIH National Cancer Institute, and Johns Hopkins Hospital. The software also is being evaluated on patients. The targeting accuracy of the system was evaluated using 3D Slicer and the NA-MIC Kit [[{{fullurl:{{FULLPAGENAME}}}}#ref_2 2], [{{fullurl:{{FULLPAGENAME}}}}#ref_5 5], [{{fullurl:{{FULLPAGENAME}}}}#ref_6 6], [{{fullurl:{{FULLPAGENAME}}}}#ref_7 7]]. |
[[{{fullurl:{{FULLPAGENAME}}}}#ref_15 15], [{{fullurl:{{FULLPAGENAME}}}}#ref_18 18], [{{fullurl:{{FULLPAGENAME}}}}#ref_16 16]] | [[{{fullurl:{{FULLPAGENAME}}}}#ref_15 15], [{{fullurl:{{FULLPAGENAME}}}}#ref_18 18], [{{fullurl:{{FULLPAGENAME}}}}#ref_16 16]] | ||
| Line 20: | Line 20: | ||
=Software= | =Software= | ||
| − | *[ | + | *[https://www.slicer.org/wiki/Modules:ProstateNav-Documentation-3.6 ProstateNav]: MRI-guided prostate biopsy module with multiple device support |
** Implements a complete MRI-guided biopsy workflow: calibration, planning, targeting, verification | ** Implements a complete MRI-guided biopsy workflow: calibration, planning, targeting, verification | ||
** Supports multiple devices: transrectal robot, transperineal template, transperineal robot | ** Supports multiple devices: transrectal robot, transperineal template, transperineal robot | ||
| Line 37: | Line 37: | ||
=Related pages= | =Related pages= | ||
*[http://www.slicer.org/pages/Special:SlicerDownloads Slicer 3.6 download] | *[http://www.slicer.org/pages/Special:SlicerDownloads Slicer 3.6 download] | ||
| − | *[ | + | *[https://www.slicer.org/wiki/Documentation-3.6 Slicer 3.6 documentation] |
| − | *[ | + | *[https://www.slicer.org/wiki/Modules:ProstateNav-Documentation-3.6 ProstateNav documentation] |
*[http://www.brighamandwomens.org/radiology/ Department of Radiology, Brigham and Women's Hospital and Harvard Medical School] | *[http://www.brighamandwomens.org/radiology/ Department of Radiology, Brigham and Women's Hospital and Harvard Medical School] | ||
*[http://snr.spl.harvard.edu/ Surgical Navigation and Robotics Laboratory, Image-Guided Therapy Program, Brigham and Women's Hospital and Harvard Medical School] | *[http://snr.spl.harvard.edu/ Surgical Navigation and Robotics Laboratory, Image-Guided Therapy Program, Brigham and Women's Hospital and Harvard Medical School] | ||
Latest revision as of 18:07, 10 July 2017
Home < DBP2:QueensFinal:2010back to DBP2 Main
Overview
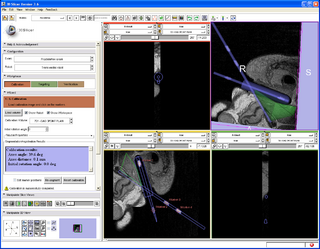
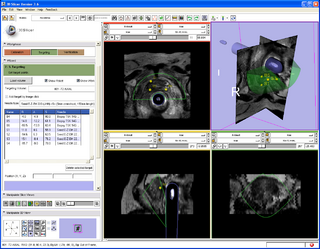
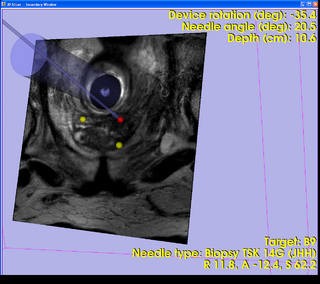
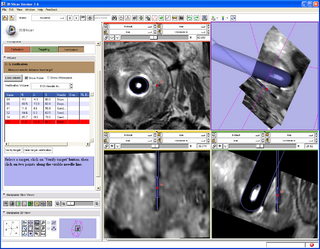
A 3D Slicer based end-to-end application was developed for MRI-guided prostate biopsy (implemented as a 3D Slicer 3.6 module, ProstateNav). The application supports multiple targeting devices (transrectal robot [5, 15], transperineal template, and transperineal robot [17, 18]) and multiple needle types (for biopsy and seed placement). Automatic and semi-automatic registration of the targeting device to the planning image coordinate system was implemented using 4 line fiducials or 3 orthogonal Z-shaped fiducials [15, 16, 18]. During the planning phase, the clinician defines several point targets for biopsy and seed placement. The 3D Slicer platform provides flexible visualization of several diagnostic image types (T2, contrast-enhanced, etc.). In the targeting phase, the software computes targeting parameters for each site that permit the targeting devices to accurately position the needles. The software offers a means to quantitatively assess the accuracy of needle placement. Patient motion during the intervention (dislocation and deformation between the planning and verification images) can be easily visualized, thereby reducing the chance of incorrect needle placement during lengthy procedures. The software is being tested in phantom experiments at three clinical sites: Brigham and Women's Hospital, NIH National Cancer Institute, and Johns Hopkins Hospital. The software also is being evaluated on patients. The targeting accuracy of the system was evaluated using 3D Slicer and the NA-MIC Kit [2, 5, 6, 7]. [15, 18, 16]
Preliminary prostate segmentation and registration algorithms were developed. The initial results were encouraging and have been published in journals and presented at conferences. Current algorithm implementations are stored in the NA-MIC sandbox repository. After optimization and tuning they will be to be added to the clinical application [1, 8, 11, 19]. A fast patient motion detection algorithm, based on slice-to-volume registration, also was developed using the NA-MIC Kit [3, 4, 9, 14].
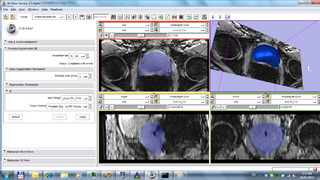
Applicability of 3D Slicer as a generic computer-assisted intervention platform was evaluated. Several architectural and usability features were identified and implemented that enable effective use of 3D Slicer in the operating room [10, 12, 13, 15, 16, 17, 18].
Utilization and further enhancement of the end results are planned in the scope of a new collaboration between NA-MIC/OCAIRO collaboration and through other research grants (applications pending).
Software
- ProstateNav: MRI-guided prostate biopsy module with multiple device support
- Implements a complete MRI-guided biopsy workflow: calibration, planning, targeting, verification
- Supports multiple devices: transrectal robot, transperineal template, transperineal robot
- Provides 3D visualization of patient images, targets, devices in the control and scanner room
- Latest stable version is available in Slicer 3.6.1 release. Latest development version source code is available in the NA-MIC Sandbox
- Tutorial: presentation, dataset
Listing and short description of the sample data
- Clinical data sets of MRI-guided prostate biopsies
- 63 anonymized prostate MRI sequences of 5 patients (Pt*), acquired at NIH-NCI, PI-s: Camphausen, Kausal and Pinto.
- Imaging sessions: (date).Diag = diagnostic; (date).B(id), (date).LR(id), (date).HR(id) = trans-rectal prostate biopsy
- Image types: Needle Ax = needle insertion confirmation image; SAG 3POINT PLAN = calibration image
- Tutorial data set
- One clinical dataset of robot-assisted trans-rectal prostate biopsy, contains calibration, planning, and verification images
Related pages
- Slicer 3.6 download
- Slicer 3.6 documentation
- ProstateNav documentation
- Department of Radiology, Brigham and Women's Hospital and Harvard Medical School
- Surgical Navigation and Robotics Laboratory, Image-Guided Therapy Program, Brigham and Women's Hospital and Harvard Medical School
- Radiation Oncology Branch, National Cancer Institute
- Department of Radiology, Johns Hopkins Hospital
- Laboratory for Percutaneous Surgery, Queen's University
References
- Gao Y., Sandhu R., Fichtinger G., Tannenbaum A.R. A Coupled Global Registration and Segmentation Framework with Application to Magnetic Resonance Prostate Imagery. IEEE Trans Med Imaging. 2010 Oct;29(10):1781-94. PMID: 20529727.
- Xu H., Lasso A., Vikal S., Guion P., Krieger A., Kaushal A., Whitcomb L.L., Fichtinger G. MRI-guided Robotic Prostate Biopsy: A Clinical Accuracy Validation. Med Image Comput Comput Assist Interv. 2010;13(Pt 3):383-91. PMID: 20879423.
- Tadayyon, H., A. Lasso, S. Gill, A. Kaushal, P. Guion, and G. Fichtinger. "Target Motion Compensation in MRI-guided Prostate Biopsy with Static Images", EMBC2010 - 32nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Buenos Aires, Argentina, pp. 5416-5419, 2010.
- Lasso, A., S. Avni, and G. Fichtinger. "Targeting Error Simulator for Image-guided Prostate Needle Placement", EMBC2010 - 32nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Buenos Aires, Argentina, pp. 5424-5427, 2010.
- Xu, H., A. Lasso, S. Vikal, P. Guion, A. Krieger, A. Kaushal, L. Whitcomb, and G. Fichtinger. "MRI-Guided Transrectal Robotic Prostate Biopsy Validation", The American Association of Physicists in Medicine (AAPM) Annual Meeting 2010, July 18-22, vol. 37, 3128 (2010), Philadelphia, Pennsylvania, pp. 3128-3129, 2010.
- Xu, H., A. Lasso, S. Vikal, P. Guion, A. Krieger, A. Kaushal, L. Whitcomb, and G. Fichtinger. "Clinical Accuracy of Robot-Assisted Prostate Biopsy in Closed MRI Scanner", The Hamlyn Symposium on Medical Robotics, The Royal Society, London, UK, 25 May 2010.
- Xu, H., A. Lasso, S. Vikal, P. Guion, A. Krieger, A. Kaushal, L. L. Whitcomb, and G. Fichtinger. "Accuracy validation for MRI-guided robotic prostate biopsy", Medical Imaging 2010: Visualization, Image-Guided Procedures, and Modeling, San Diego, California, USA, SPIE, pp. 762517-762517-8, 2010.
- Gao, Y., A. Tannenbaum. "Shape based MRI prostate image segmentation using local information driven directional distance Bayesian method", Medical Imaging 2010: Visualization, Image-Guided Procedures, and Modeling, San Diego, California, USA, SPIE, pp. 762308-1 - 762308-9, 2010.
- Tadayyon, H., G. Fichtinger, A. Lasso, S. Vikal, and S. Gill. "MRI-Guided prostate motion tracking by means of multislice-to-volume registration", Proc. SPIE,(2010); Vol. 7625, 76252V.
- Lasso, A., J. Tokuda, S. Vikal, C. M. Tempany, N. Hata, and G. Fichtinger. "A generic computer assisted intervention plug-in module for 3D Slicer with multiple device support." Int Conf Med Image Comput Comput Assist Interv. 2009;
- Vikal S., Haker S., Tempany C.M., Fichtinger G. Prostate Contouring in MRI Guided Biopsy. Proceedings of SPIE Medical Imaging, Image Processing 2009; 7259.
- Boisvert, J., D. Gobbi, S. Vikal, R. Rohling, G. Fichtinger, and P. Abolmaesumi. "An open-source solution for interactive acquisition, processing and transfer of interventional ultrasound images." Workshop on Systems and Architectures for Computer Assisted Interventions, held in conjunction with the 11th International Conference on Medical Image Computing and Computer Assisted Intervention, 2008.
- Fischer G.S., Krieger A., Iordachita I., Csoma C., Whitcomb L., Fichtinger G. MRI Compatibility of Robot Actuation Techniques - A Comparative Study. Int Conf Med Image Comput Comput Assist Interv. 2008;11(Pt 2):509-517. PMID: 18982643.
- Gill S., Abolmaesumi P., Vikal S., Mousavi P., Fichtinger G. Intraoperative Prostate Tracking with Slice-to-Volume Registration in MRI. Proceedings of the 20th International Conference of the Society for Medical Innovation and Technology 2008; 154-158.
- Krieger, A., P. Guion, C. Csoma, I. Iordachita, A. Singh, A. Kaushal, C. Menard, G. Fichtinger, and L. Whitcomb. "Design and Preliminary Clinical Studies of an MRI-Guided Transrectal Prostate Intervention System." International Society of Magnetic Resonance in Medicine (ISMRM), 2008.
- Mewes P., Tokuda J., DiMaio S.P., Fischer G., Csoma C., Gobbi D., Tempany C.M., Fichtinger G., Hata N. Integrated System for Robot-Assisted in Prostate Biopsy in Closed MRI Scanner. Proceedings of the IEEE International Conference on Robotics and Automation 2008; 2959-2962.
- Tokuda, J., S. DiMaio, G. Fischer, C. Csoma, D. Gobbi, G. Fichtinger, N. Hata, and C. Tempany. "Real-time MR Imaging Controlled by Transperineal Needle Placement Device for MRI-guided Prostate Biopsy", 16th Scientific Meeting and Exhibition of International Society of Magnetic Resonance in Medicine, 2008.
- Tokuda J., Fischer G.S., Csoma C., DiMaio S.P., Gobbi D.G., Fichtinger G., Tempany C.M., Hata N. Software Strategy for Robotic Transperineal Prostate Therapy in Closed-Bore MRI. Int Conf Med Image Comput Comput Assist Interv. 2008;11(Pt 2):701-709. PMID: 18982666. PMCID: PMC2692941.
- Vikal, S., S. Haker, C. Tempany, and G. Fichtinger. Prostate contouring in MRI guided biopsy.Proceedings of SPIE Medical Imaging, Image Processing 2009; 7259.