Difference between revisions of "Projects:NonparametricSegmentation"
| Line 1: | Line 1: | ||
| − | Back to [[NA-MIC_Internal_Collaborations:StructuralImageAnalysis|NA-MIC Collaborations]], [[Algorithm:MIT|MIT Algorithms]], | + | Back to [[NA-MIC_Internal_Collaborations:StructuralImageAnalysis|NA-MIC Collaborations]], [[Algorithm:MIT|MIT Algorithms]], |
__NOTOC__ | __NOTOC__ | ||
| − | = | + | = Nonparametric Segmentation = |
We propose a non-parametric, probabilistic model for the automatic segmentation of medical images, given a training set of images and corresponding label maps. The resulting inference algorithms we develop rely on pairwise registrations between the test image and individual training images. The training labels are then transferred to the test image and fused to compute a final segmentation of the test subject. Label fusion methods have been shown to yield accurate segmentation, since the use | We propose a non-parametric, probabilistic model for the automatic segmentation of medical images, given a training set of images and corresponding label maps. The resulting inference algorithms we develop rely on pairwise registrations between the test image and individual training images. The training labels are then transferred to the test image and fused to compute a final segmentation of the test subject. Label fusion methods have been shown to yield accurate segmentation, since the use | ||
of multiple registrations captures greater inter-subject anatomical variability and improves robustness against occasional registration failures, cf. [1,2,3]. To the best of our knowledge, this project investigates the first comprehensive probabilistic framework that rigorously motivates label fusion as a segmentation approach. The proposed framework allows us to compare different label fusion algorithms theoretically and practically. In particular, recent label fusion or multi-atlas segmentation algorithms are interpreted as special cases of our framework. | of multiple registrations captures greater inter-subject anatomical variability and improves robustness against occasional registration failures, cf. [1,2,3]. To the best of our knowledge, this project investigates the first comprehensive probabilistic framework that rigorously motivates label fusion as a segmentation approach. The proposed framework allows us to compare different label fusion algorithms theoretically and practically. In particular, recent label fusion or multi-atlas segmentation algorithms are interpreted as special cases of our framework. | ||
| − | = | + | = Description = |
We instantiate our model in the context of brain MRI segmentation, where whole brain MRI volumes are automatically parcellated into the following anatomical Regions of Interest (ROI): white matter (WM), cerebral cortex(CT), lateral ventricle (LV), hippocampus(HP), amygdala (AM), thalamus (TH), caudate (CA), putamen (PU), palladum (PA). | We instantiate our model in the context of brain MRI segmentation, where whole brain MRI volumes are automatically parcellated into the following anatomical Regions of Interest (ROI): white matter (WM), cerebral cortex(CT), lateral ventricle (LV), hippocampus(HP), amygdala (AM), thalamus (TH), caudate (CA), putamen (PU), palladum (PA). | ||
The proposed non-parametric model yields four types of label fusion algorithms: | The proposed non-parametric model yields four types of label fusion algorithms: | ||
| Line 22: | Line 22: | ||
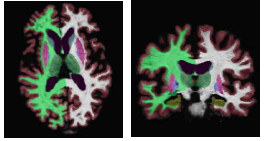
[[File:Segmentation_example2.png]] | [[File:Segmentation_example2.png]] | ||
| − | =Experiments= | + | == Experiments == |
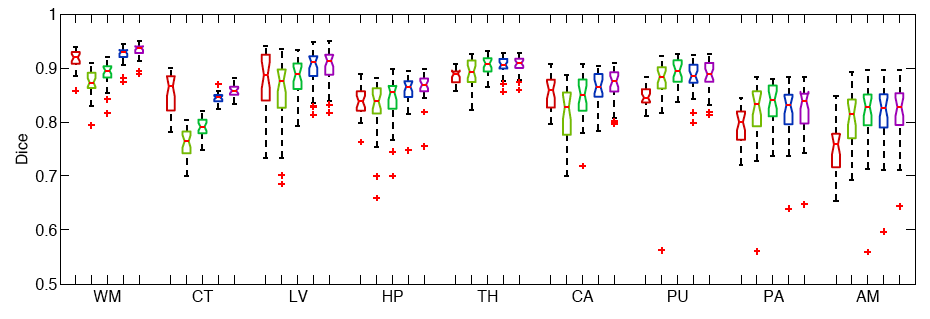
We conduct two sets of experiments to validate our framework. In the first set of experiments, we use 39 brain MRI scans – with manually segmented white matter, cerebral cortex, ventricles and subcortical structures – to compare different label fusion algorithms and the widely-used Freesurfer whole-brain segmentation tool [4]. | We conduct two sets of experiments to validate our framework. In the first set of experiments, we use 39 brain MRI scans – with manually segmented white matter, cerebral cortex, ventricles and subcortical structures – to compare different label fusion algorithms and the widely-used Freesurfer whole-brain segmentation tool [4]. | ||
| Line 37: | Line 37: | ||
| − | =Conclusion= | + | == Conclusion == |
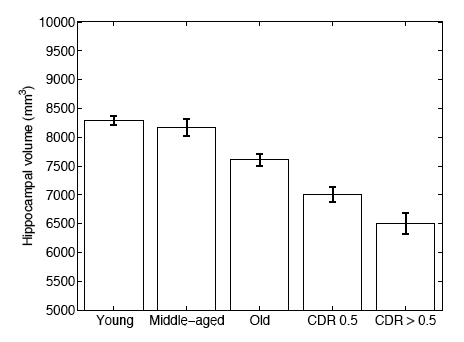
In this work, we investigate a generative model that leads to label fusion style image segmentation methods. Within the proposed framework, we derived various algorithms that combine transferred training labels into a single segmentation estimate. With a dataset of 39 brain MRI scans and corresponding label maps obtained from an expert, we experimentally compared these segmentation algorithms with Freesurfer’s widely-used atlas-based segmentation tool [4]. Our results suggested that the proposed framework yields accurate and robust segmentation tools that can be employed on large multi-subject datasets. In a second experiment, we employed one of the developed segmentation algorithms to compute hippocampal volumes in MRI scans of 304 subjects. A comparison of these measurements across clinical and age groups indicate that the proposed algorithms are sufficiently sensitive to detect hippocampal atrophy that precedes probable onset of Alzheimer’s Disease. | In this work, we investigate a generative model that leads to label fusion style image segmentation methods. Within the proposed framework, we derived various algorithms that combine transferred training labels into a single segmentation estimate. With a dataset of 39 brain MRI scans and corresponding label maps obtained from an expert, we experimentally compared these segmentation algorithms with Freesurfer’s widely-used atlas-based segmentation tool [4]. Our results suggested that the proposed framework yields accurate and robust segmentation tools that can be employed on large multi-subject datasets. In a second experiment, we employed one of the developed segmentation algorithms to compute hippocampal volumes in MRI scans of 304 subjects. A comparison of these measurements across clinical and age groups indicate that the proposed algorithms are sufficiently sensitive to detect hippocampal atrophy that precedes probable onset of Alzheimer’s Disease. | ||
| − | =Publications= | + | == Literature == |
| + | [1] X. Artaechevarria, A. Munoz-Barrutia, and C. Ortiz de Solorzano. Combination strategies in multi-atlas image segmentation: | ||
| + | Application to brain MR data. IEEE Tran. Med. Imaging, 28(8):1266 – 1277, 2009. | ||
| + | |||
| + | [2] R.A. Heckemann, J.V. Hajnal, P. Aljabar, D. Rueckert, and A. Hammers. Automatic anatomical brain MRI segmentation | ||
| + | combining label propagation and decision fusion. Neuroimage, 33(1):115–126, 2006. | ||
| + | |||
| + | [3] T. Rohlfing, R. Brandt, R. Menzel, and C.R. Maurer. Evaluation of atlas selection strategies for atlas-based image | ||
| + | segmentation with application to confocal microscopy images of bee brains. NeuroImage, 21(4):1428–1442, 2004. | ||
| + | |||
| + | [4] Freesurfer Wiki. http://surfer.nmr.mgh.harvard.edu. | ||
| + | |||
| + | = Publications = | ||
| + | '' In Press '' | ||
| + | |||
<font color="red">'''New: '''</font> Supervised Nonparametric Image Parcellation, M.R. Sabuncu, B.T. Thomas Yeo, K. Van Leemput, B. Fischl, and P. Golland. To appear in Proceedings of the International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI), 2009. | <font color="red">'''New: '''</font> Supervised Nonparametric Image Parcellation, M.R. Sabuncu, B.T. Thomas Yeo, K. Van Leemput, B. Fischl, and P. Golland. To appear in Proceedings of the International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI), 2009. | ||
| Line 51: | Line 65: | ||
*Harvard: Koen Van Leemput and Bruce Fischl | *Harvard: Koen Van Leemput and Bruce Fischl | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Revision as of 19:48, 20 October 2009
Home < Projects:NonparametricSegmentationBack to NA-MIC Collaborations, MIT Algorithms,
Nonparametric Segmentation
We propose a non-parametric, probabilistic model for the automatic segmentation of medical images, given a training set of images and corresponding label maps. The resulting inference algorithms we develop rely on pairwise registrations between the test image and individual training images. The training labels are then transferred to the test image and fused to compute a final segmentation of the test subject. Label fusion methods have been shown to yield accurate segmentation, since the use of multiple registrations captures greater inter-subject anatomical variability and improves robustness against occasional registration failures, cf. [1,2,3]. To the best of our knowledge, this project investigates the first comprehensive probabilistic framework that rigorously motivates label fusion as a segmentation approach. The proposed framework allows us to compare different label fusion algorithms theoretically and practically. In particular, recent label fusion or multi-atlas segmentation algorithms are interpreted as special cases of our framework.
Description
We instantiate our model in the context of brain MRI segmentation, where whole brain MRI volumes are automatically parcellated into the following anatomical Regions of Interest (ROI): white matter (WM), cerebral cortex(CT), lateral ventricle (LV), hippocampus(HP), amygdala (AM), thalamus (TH), caudate (CA), putamen (PU), palladum (PA). The proposed non-parametric model yields four types of label fusion algorithms:
(1) Majority Voting: The algorithm computes the most frequent propagated labels at each voxel independently. This is a segmentation algorithm commonly used in practice, e.g. [2,3].
(2) Local Label Fusion: An independent weighted averaging of propagated labels, where the weights vary at each voxel and are a function of the intensity difference between the training image and test image.
(3) Semi-Local Label Fusion: Propagated labels are fused in a weighted fashion using a variational mean field algorithm. The weights are encouraged to be similar in local patches.
(4) Global Label Fusion: Propagated labels are fused in a weighted fashion using an Expectation Maximization algorithm. The weights are global, i.e., there is a single weight for each training subject.
The following figure shows an example segmentation obtained via Local Label Fusion.
Experiments
We conduct two sets of experiments to validate our framework. In the first set of experiments, we use 39 brain MRI scans – with manually segmented white matter, cerebral cortex, ventricles and subcortical structures – to compare different label fusion algorithms and the widely-used Freesurfer whole-brain segmentation tool [4].
The following figure shows the Boxplots of Dice scores for all methods: Freesurfer (red), Majority Voting (yellow), Global Weighted Fusion (green), Local Weighted Voting (blue), Semi-local Weighted Fusion (purple).
Our results indicate that the proposed framework yields more accurate segmentation than Freesurfer and Majority Voting.
In a second experiment, we use brain MRI scans of 304 subjects to demonstrate that the proposed segmentation tool is sufficiently sensitive to robustly detect hippocampal atrophy that foreshadows the onset of Alzheimer’s Disease. The following figure plots the Average Hippocampal volumes for five different groups (young: younger than 30, middle aged: older than 30, younger than 60; old: older than 60; patients with MCI, and AD patients) in the 304 subjects of Experiment 2. Error bars indicate standard error.
Conclusion
In this work, we investigate a generative model that leads to label fusion style image segmentation methods. Within the proposed framework, we derived various algorithms that combine transferred training labels into a single segmentation estimate. With a dataset of 39 brain MRI scans and corresponding label maps obtained from an expert, we experimentally compared these segmentation algorithms with Freesurfer’s widely-used atlas-based segmentation tool [4]. Our results suggested that the proposed framework yields accurate and robust segmentation tools that can be employed on large multi-subject datasets. In a second experiment, we employed one of the developed segmentation algorithms to compute hippocampal volumes in MRI scans of 304 subjects. A comparison of these measurements across clinical and age groups indicate that the proposed algorithms are sufficiently sensitive to detect hippocampal atrophy that precedes probable onset of Alzheimer’s Disease.
Literature
[1] X. Artaechevarria, A. Munoz-Barrutia, and C. Ortiz de Solorzano. Combination strategies in multi-atlas image segmentation: Application to brain MR data. IEEE Tran. Med. Imaging, 28(8):1266 – 1277, 2009.
[2] R.A. Heckemann, J.V. Hajnal, P. Aljabar, D. Rueckert, and A. Hammers. Automatic anatomical brain MRI segmentation combining label propagation and decision fusion. Neuroimage, 33(1):115–126, 2006.
[3] T. Rohlfing, R. Brandt, R. Menzel, and C.R. Maurer. Evaluation of atlas selection strategies for atlas-based image segmentation with application to confocal microscopy images of bee brains. NeuroImage, 21(4):1428–1442, 2004.
[4] Freesurfer Wiki. http://surfer.nmr.mgh.harvard.edu.
Publications
In Press
New: Supervised Nonparametric Image Parcellation, M.R. Sabuncu, B.T. Thomas Yeo, K. Van Leemput, B. Fischl, and P. Golland. To appear in Proceedings of the International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI), 2009.
New: Nonparametric Mixture Models for Supervised Image Parcellation, M.R. Sabuncu, B.T.T. Yeo, K. Van Leemput, B. Fischl, and P. Golland. To be presented at PMMIA Workshop at MICCAI 2009.
Key Investigators
- MIT: Mert R. Sabuncu, B.T. Thomas Yeo, Koen Van Leemput and Polina Golland
- Harvard: Koen Van Leemput and Bruce Fischl