Difference between revisions of "Collaboration: COPDGene"
| (6 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | Back to [[NA-MIC_External_Collaborations]] | + | Back to [[NA-MIC_External_Collaborations|NA-MIC External Collaborations]] |
| − | + | __NOTOC__ | |
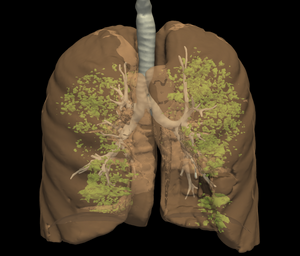
[[Image:LungCover-LowRes.png|thumb|right|300px|Burden of emphysema in a COPD subject]] | [[Image:LungCover-LowRes.png|thumb|right|300px|Burden of emphysema in a COPD subject]] | ||
| Line 13: | Line 13: | ||
== Specific Aims == | == Specific Aims == | ||
| − | * Specific Aim 1: Cohort Building. The enrollment of 10,000 subjects is balanced with 2/3 non-Hispanic White and 1/3 African American, distributed across the full spectrum of disease severity and both genders | + | * Specific Aim 1: Cohort Building. The enrollment of 10,000 subjects is balanced with 2/3 non-Hispanic White and 1/3 African American, distributed across the full spectrum of disease severity and both genders. The cohort is specifically being recruited for a genome wide association study (GWAS) analysis and is large enough to provide adequate statistical power to detect genes exerting modest effects on risk. |
* Specific Aim 2: Characterization of Subtypes of COPD. The main characterization of COPD subtypes will be based on the presence and severity of parenchymal and airway disease based on inspiratory and expiratory high-resolution chest CT scans. | * Specific Aim 2: Characterization of Subtypes of COPD. The main characterization of COPD subtypes will be based on the presence and severity of parenchymal and airway disease based on inspiratory and expiratory high-resolution chest CT scans. | ||
| − | * Specific Aim3: Genome-Wide Association Study | + | * Specific Aim3: Genome-Wide Association Study. The initial study plan for the GWAS involves four phases. There will be an initial GWAS on a balanced group of 3000 subjects of current or former smoker case and control subjects (2000 White and 1000 African American) in Phase 1. Statistical signals (SNPs in or between genes) identified in Phase I will be confirmed in Phase II with a custom SNP array that will provide greater coverage of genes. In Phase III SNPs in genes/regions identified and confirmed in Phases I and II will be investigated with regional fine mapping and tests of associations to identify causal genes. The final group of candidate genes will be replicated in other COPD cohorts as Phase IV. With continued improvements in SNP genotyping technology additional phases (beyond Phase 1) may be analyzed at the genome-wide level. |
* Specific Aim 4: Natural history of COPD and Risk Factors for Progression. The COPDGene cohort will be established for longitudinal follow-up with regular contact made to determine mortality, comorbid disease events and disease status based on clinical and/or chest CT evidence of progression. | * Specific Aim 4: Natural history of COPD and Risk Factors for Progression. The COPDGene cohort will be established for longitudinal follow-up with regular contact made to determine mortality, comorbid disease events and disease status based on clinical and/or chest CT evidence of progression. | ||
| Line 30: | Line 30: | ||
Finally, the wealth of data to be accrued in COPDGene® will be stored and made available to the broader scientific community for future studies. This will include the detailed phenotypic subject information, whole genome data and the imaging data from CT scans. | Finally, the wealth of data to be accrued in COPDGene® will be stored and made available to the broader scientific community for future studies. This will include the detailed phenotypic subject information, whole genome data and the imaging data from CT scans. | ||
| − | == Grant #== | + | == Grant#== |
| − | The project described is being supported by Award Numbers U01HL089897 and U01HL089856 from the National Heart, Lung, And Blood Institute. | + | The project described is being supported by Award Numbers U01HL089897 and U01HL089856 from the National Heart, Lung, And Blood Institute. |
| + | ==Key Personnel== | ||
| + | *National Jewish Health: James D. Crapo (PI) | ||
| + | *Brigham and Women's Hospital: Edwin K. Silverman (PI) | ||
| + | *NA-MIC: Raul San Jose, James Ross | ||
| + | ==Grant Duration== | ||
| + | 09/27/2007-07/31/2012 | ||
== References == | == References == | ||
# Cohen BH, Ball WC Jr, Brashears S et al. Risk factors in chronic obstructive pulmonary disease (COPD). Am J Epidemiol 1977; 105(3):223-32 | # Cohen BH, Ball WC Jr, Brashears S et al. Risk factors in chronic obstructive pulmonary disease (COPD). Am J Epidemiol 1977; 105(3):223-32 | ||
| Line 38: | Line 44: | ||
# Friedlander AL, Lynch D, Dyar LA et al. Phenotypes of chronic obstructive pulmonary disease. COPD 2007; 4(4):355-84 | # Friedlander AL, Lynch D, Dyar LA et al. Phenotypes of chronic obstructive pulmonary disease. COPD 2007; 4(4):355-84 | ||
# Gottlieb DJ, Wilk JB, Harmon M et al. Heritability of longitudinal change in lung function. The Framingham study. Am J Respir Crit Care Med 2001; 164(9):1655-9 | # Gottlieb DJ, Wilk JB, Harmon M et al. Heritability of longitudinal change in lung function. The Framingham study. Am J Respir Crit Care Med 2001; 164(9):1655-9 | ||
| − | # Hersh CP, | + | # Hersh CP, DeMeo DL, Lazarus R et al. Genetic association analysis of functional impairment in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 173(9):977-84 |
| − | # Hersh CP, DeMeo DL, Reilly JJ | + | # Hersh CP, DeMeo DL, Reilly JJ, Silverman EK. Xenobiotic metabolizing enzyme gene polymorphisms predict response to lung volume reduction surgery. Respir Res. 2007 Aug 8;8:59. PubMed PMID: 17686149; PubMed Central PMCID: PMC2048957. |
| − | # Hoffman EA, Simon BA, McLennan G. State of the Art. A structural and functional assessment of the lung via multidetector-row computed tomography: phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006; 3(6):519-32 | + | # Hoffman EA, Simon BA, McLennan G. State of the Art. A structural and functional assessment of the lung via multidetector-row computed tomography: phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006 Aug;3(6):519-32. Review. PubMed PMID: 16921136; PubMed Central PMCID: PMC2647643. |
| − | # Hoyert DL, Arias E, Smith BL | + | # Hoyert DL, Arias E, Smith BL, Murphy SL, Kochanek KD. Deaths: final data for 1999. Natl Vital Stat Rep. 2001 Sep 21;49(8):1-113. PubMed PMID: 11591077. |
| − | # Kueppers F, Miller RD, Gordon H et al. Familial prevalence of chronic obstructive pulmonary disease in a matched pair study. Am J Med 1977; 63(3):336-42 | + | # Kueppers F, Miller RD, Gordon H et al. Familial prevalence of chronic obstructive pulmonary disease in a matched pair study. Am J Med. 1977 Sep;63(3):336-42. PMID: 302643. |
# Lokke A, Lange P, Scharling H et al. Developing COPD: a 25 year follow up study of the general population. Thorax 2006; 61(11):935-9 | # Lokke A, Lange P, Scharling H et al. Developing COPD: a 25 year follow up study of the general population. Thorax 2006; 61(11):935-9 | ||
# Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370(9589):765-73 | # Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370(9589):765-73 | ||
Latest revision as of 21:17, 13 December 2016
Home < Collaboration: COPDGeneBack to NA-MIC External Collaborations
Abstract
Chronic Obstructive Pulmonary Disease (COPD) is the fourth leading cause of death in the United States and an important public health issue [8]. An estimated 24 million individuals in the U.S. may be affected by COPD [12]. Both the number of affected individuals and the number of deaths from COPD are expected to increase as the population ages [11]. COPD is a heterogeneous condition, with a variety of disease-related phenotypes [3,16]. Better understanding of the disease mechanisms is needed to develop effective treatments and prevention strategies. To accomplish this, we need improved understanding of the etiology of COPD, clinical classifications of the disease that are biologically and medically coherent, and knowledge of genetic factors that influence risk of COPD. COPD is strongly associated with smoking, but only a minority of smokers will develop COPD, suggesting that there may be unique genetic differences among individuals leading to greater susceptibility to the most adverse effects of cigarette smoke in some individuals [10]. Relatives of COPD patients show an increased prevalence of airflow obstruction, which supports a role for genetic factors predisposing smokers to COPD [1,9,13]. Smokers with first degree relatives affected by COPD have two to three times the risk of developing disease [13,17]. Genetic factors have been associated with response to lung volume reduction surgery [6] as well as specific patterns of emphysema [2] and degree of functional impairment [5]. Estimated heritability for decline in lung function with age using parent-offspring pairs who both smoke are 0.18 for FEV1 and 0.39 for FVC [4]. Because COPD is likely the result of multiple genes, some of which may interact with environment risk factors (primarily smoking), estimates of heritability that do not include the effects of smoking on lung function are likely to underestimate the true genetic component in COPD. The Genetic Epidemiology of COPD (COPDGene®) Study was designed to identify genetic factors in COPD, to define and characterize disease-related phenotypes and to assess the association of disease-related phenotypes with the identified susceptibility genes. This multi-center study is funded by the National Heart, Lung and Blood Institute (NHLBI). A key feature of the project is to enroll a large cohort (10,000) of subjects, spanning the breadth of disease severity including smokers and non-smokers without COPD as controls. Two groups are being studied: Non-Hispanic Whites and Non-Hispanic African Americans.
The primary goals of the study are: 1) Phenotypic characterization of COPD subjects using computed tomography, as well as clinical and physiological measures, to separate the COPD syndrome into significant distinct subtypes that may be more etiologically homogeneous. 2) Utilize genome-wide association studies to provide insight into the genetic risk factors for COPD and its subtypes.
Specific Aims
- Specific Aim 1: Cohort Building. The enrollment of 10,000 subjects is balanced with 2/3 non-Hispanic White and 1/3 African American, distributed across the full spectrum of disease severity and both genders. The cohort is specifically being recruited for a genome wide association study (GWAS) analysis and is large enough to provide adequate statistical power to detect genes exerting modest effects on risk.
- Specific Aim 2: Characterization of Subtypes of COPD. The main characterization of COPD subtypes will be based on the presence and severity of parenchymal and airway disease based on inspiratory and expiratory high-resolution chest CT scans.
- Specific Aim3: Genome-Wide Association Study. The initial study plan for the GWAS involves four phases. There will be an initial GWAS on a balanced group of 3000 subjects of current or former smoker case and control subjects (2000 White and 1000 African American) in Phase 1. Statistical signals (SNPs in or between genes) identified in Phase I will be confirmed in Phase II with a custom SNP array that will provide greater coverage of genes. In Phase III SNPs in genes/regions identified and confirmed in Phases I and II will be investigated with regional fine mapping and tests of associations to identify causal genes. The final group of candidate genes will be replicated in other COPD cohorts as Phase IV. With continued improvements in SNP genotyping technology additional phases (beyond Phase 1) may be analyzed at the genome-wide level.
- Specific Aim 4: Natural history of COPD and Risk Factors for Progression. The COPDGene cohort will be established for longitudinal follow-up with regular contact made to determine mortality, comorbid disease events and disease status based on clinical and/or chest CT evidence of progression.
Population.
Imaging
CT scans are acquired using multi-detector CT scanners (at least 16 detector channels). Volumetric CT acquisitions are obtained both on full inspiration (200mAs), and at the end of normal expiration (50 mAs). Image reconstruction utilizes sub-millimeter slice thickness, with smooth and edge-enhancing algorithms. The quantitative analysis of the data is carried out by our imaging groups at National Jewish Hospital in Denver and Brigham and Women's Hospital in Boston.
Public Health Relevance
COPD is a disease with important public health implications given its often profound effects on functional capacity, quality of life and mortality. At this time there is a dearth of effective disease treatments for moderate to severe disease or effective secondary prevention strategies for early or occult disease. Further progress in these areas is hampered by the long latency period between smoking exposure and development of clinical disease, as well as by a relatively small proportion of smokers who develop symptomatic disease. Wide variation in disease expression patterns (airway disease, emphysema, extrapulmonary effects and patterns of exacerbations) may limit statistical power to detect successful results within these subsets in therapeutic trials. COPDGene® with its large population and focus on CT phenotypes proposes to define subsets of COPD that may reflect effects of specific genetic variants. Careful CT phenotyping may generate diagnostic imaging biomarkers and permit early disease identification in high risk groups. This early diagnosis of asymptomatic disease will provide new opportunities to develop prevention strategies and treatment to limit disease progression. Available treatments will also spur new efforts to encourage screening for early disease in smokers with continued emphasis on smoking cessation. The genetic associations expected from performing GWAS in this large cohort may reveal novel directions for defining disease mechanisms while advancing knowledge about basic mechanisms and also providing opportunities for treatment and prevention. Finally, the wealth of data to be accrued in COPDGene® will be stored and made available to the broader scientific community for future studies. This will include the detailed phenotypic subject information, whole genome data and the imaging data from CT scans.
Grant#
The project described is being supported by Award Numbers U01HL089897 and U01HL089856 from the National Heart, Lung, And Blood Institute.
Key Personnel
- National Jewish Health: James D. Crapo (PI)
- Brigham and Women's Hospital: Edwin K. Silverman (PI)
- NA-MIC: Raul San Jose, James Ross
Grant Duration
09/27/2007-07/31/2012
References
- Cohen BH, Ball WC Jr, Brashears S et al. Risk factors in chronic obstructive pulmonary disease (COPD). Am J Epidemiol 1977; 105(3):223-32
- DeMeo DL, Hersh CP, Hoffman EA et al. Genetic determinants of emphysema distribution in the national emphysema treatment trial. Am J Respir Crit Care Med 2007; 176(1):42-8
- Friedlander AL, Lynch D, Dyar LA et al. Phenotypes of chronic obstructive pulmonary disease. COPD 2007; 4(4):355-84
- Gottlieb DJ, Wilk JB, Harmon M et al. Heritability of longitudinal change in lung function. The Framingham study. Am J Respir Crit Care Med 2001; 164(9):1655-9
- Hersh CP, DeMeo DL, Lazarus R et al. Genetic association analysis of functional impairment in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 173(9):977-84
- Hersh CP, DeMeo DL, Reilly JJ, Silverman EK. Xenobiotic metabolizing enzyme gene polymorphisms predict response to lung volume reduction surgery. Respir Res. 2007 Aug 8;8:59. PubMed PMID: 17686149; PubMed Central PMCID: PMC2048957.
- Hoffman EA, Simon BA, McLennan G. State of the Art. A structural and functional assessment of the lung via multidetector-row computed tomography: phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006 Aug;3(6):519-32. Review. PubMed PMID: 16921136; PubMed Central PMCID: PMC2647643.
- Hoyert DL, Arias E, Smith BL, Murphy SL, Kochanek KD. Deaths: final data for 1999. Natl Vital Stat Rep. 2001 Sep 21;49(8):1-113. PubMed PMID: 11591077.
- Kueppers F, Miller RD, Gordon H et al. Familial prevalence of chronic obstructive pulmonary disease in a matched pair study. Am J Med. 1977 Sep;63(3):336-42. PMID: 302643.
- Lokke A, Lange P, Scharling H et al. Developing COPD: a 25 year follow up study of the general population. Thorax 2006; 61(11):935-9
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370(9589):765-73
- Mannino DM, Homa DM, Akinbami LJ et al. Chronic obstructive pulmonary disease surveillance--United States, 1971-2000. MMWR Surveill Summ 2002; 51(6):1-16
- McCloskey SC, Patel BD, Hinchliffe SJ et al. Siblings of patients with severe chronic obstructive pulmonary disease have a significant risk of airflow obstruction. Am J Respir Crit Care Med 2001; 164(8 Pt 1):1419-24
- Pillai SG, Ge D, Zhu G et al. A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet 2009; 5(3):e1000421
- Risch NJ, Zhang H. Mapping quantitative trait loci with extreme discordant sib pairs: sampling considerations. Am J Hum Genet 1996; 58(4):836-43
- Silverman EK. Exacerbations in chronic obstructive pulmonary disease: do they contribute to disease progression? Proc Am Thorac Soc 2007; 4(8):586-90
- Silverman EK, Chapman HA, Drazen JM et al. Genetic epidemiology of severe, early-onset chronic obstructive pulmonary disease. Risk to relatives for airflow obstruction and chronic bronchitis. Am J Respir Crit Care Med 1998; 157(6 Pt 1):1770-8
- Van Steen K, McQueen MB, Herbert A et al. Genomic screening and replication using the same data set in family-based association testing. Nat Genet 2005; 37(7):683-91