DBP:Head and Neck Cancer
ADAPTIVE RADIOTHERAPY FOR HEAD AND NECK CANCER
PI: Greg Sharp, MGH
Head and neck cancers account for about 60,000 new cancer cases per year and represent about 6% of all cancers in the United States [1]. These cancers are treated by a combination of chemotherapy, radiotherapy, and surgery. The five-year survival is approximately 50%. During a six-week regimen of radiotherapy, head and neck cancer patients often exhibit anatomic changes that affect their treatment. These changes include tumor regression or growth, changes in lymph node size, and changes in air cavities. Uncorrected, these changes can increase the risk of treatment complications or reduce treatment efficacy.
Adaptive radiotherapy addresses the problem of anatomic change by incrementally adjusting the radiotherapy plan, and is a prime example of personalized medicine. A mid-treatment adjustment is complex: it requires a new CT image, image segmentation, deformable registration, and mapping of the previously delivered dose onto the new image. This project proposes to use the NA-MIC Kit to develop a simple, practical workflow for achieving adaptive radiotherapy which can be applied on a case-by-case basis.
Specific Aims
1. Develop an open computational workflow for adaptive radiotherapy. We will develop a practical workflow for adaptive therapy planning that enables the registration of successive CT scans, segmentation of the tumor and the critical structures in CT, mapping of prior radiation plans onto new images, and planning of additional radiation therapy. We hypothesize that a flexible framework or workflow will enable an adaptive plan to be generated, reviewed, and ready for use within hours of acquisition.
2. Validate the accuracy of image analysis algorithms for radiotherapy. We will investigate and quantify the accuracy of automatic image registration and segmentation algorithms to establish spatial correspondences across consecutive CT scans and to delineate structures for radiation planning. We will adapt and compare the algorithms within NA-MIC Kit, and work with the Computer Science Core to develop novel segmentation and registration methods tailored to adaptive radiotherapy planning.
3. Evaluate the dosimetric gain of adaptive radiotherapy. Using CT images acquired from patients before treatment and at the mid-point during treatment, we will perform dosimetric comparisons of traditional radiotherapy and adaptive radiotherapy. We hypothesize that adaptive radiotherapy will result in a clinical gain in the probability of tumor control and/or a reduction in complication rate, as predicted by radiation dose-response models.
This project features three innovations: (1) It will establish the feasibility of an open-source software platform for adaptive radiotherapy; (2) optimize the platform for radiotherapy; and (3) evaluate the clinical gain through dosimetric comparison. Our aims support the NIH-funded project “Proton radiation therapy research” (2 P01 CA21239-29A, DeLaney PI) by providing image analysis tools for adaptive proton therapy. The clinical hypothesis of the supported project is that proton-beam radiotherapy improves the therapeutic ratio between cure probability and complication risk in non-small cell lung cancer, liver tumors, pediatric medulloblastoma and rhabdomyosarcoma, spine/skull base sarcomas, and paranasal sinus malignancies. NA-MIC will play a critical role in the project by bringing adaptive radiotherapy to proton treatments of the paranasal sinus. NA-MIC is uniquely positioned to provide user-ready software and state-of-the-art imaging algorithms.
Background
Anatomic changes during radiotherapy can cause the clinical target volume to move outside of the previously planned treatment field, or conversely, can cause critical structures such as the parotid gland, spinal cord, and brainstem to move into the treatment field. Adaptive radiotherapy has been explored for photon therapy [2,3], but the technique is not yet widely used. The need for adaptive radiotherapy is gaining in importance as the use of more aggressive chemotherapy increases [4]. Adaptive radiotherapy, may be especially important for proton therapy. Protons have a finite range of penetration and treatment accuracy depends upon both lateral positioning and target depth.
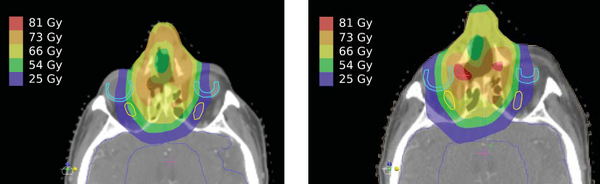
Figure 1 shows that the original treatment plan (left) has a uniform target distribution and good sparing of critical organs. At mid-treatment (right), there is an anatomic shift, which results in undesirable hot spots and a high dose to the right optic nerve and retina. To date, there have been no studies on the effect of anatomic change for proton-beam treatments in the head and neck. This study will be the first to assess whether plan adaptation can improve proton treatments for patients.
One of the reasons that adaptive radiotherapy has not become widespread is that dose planning requires expert training and sophisticated software. The additional treatment planning required to account for anatomical changes is considered too costly in terms of clinical time. Automated adaptation of the plan promises to alleviate this problem, but current systems still require signifi cant time investment per case, making it difficult to evaluate the impact of different algorithmic strategies. The NA-MIC Kit will serve a critical role in making adaptive therapy a practical reality. 3D Slicer will be used as the imaging platform for registration and segmentation of longitudinal CT images, as well as for clinical visualization and review.
Investigators
| NAME | DEGREE | INSTITUTION | EXPERIENCE | ROLE |
| GREG SHARP | PH.D. | MGH | MEDICAL PHYSICIST | DBP PI |
| ANNIE CHAN | M.D. | MGH | RADIATION ONCOLOGIST | CLINICAL EVALUATION |
| STEVE PIEPER | PH.D. | ISOMICS | SOFTWARE ENGINEERING | LEADING ENGINEERING CONTACT |
| POLINA GOLLAND | PH.D. | MIT | COMPUTER SCIENTIST | LEAD ALGORITHMS CONTACT |
Methods
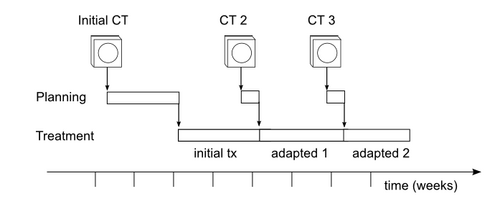
Aim 1. We will define a workflow for adaptive radiotherapy that uses the NA-MIC Kit together with existing commercial treatment planning software (TPS). TPS will be used to create the initial treatment plan. Using one or more additional CT scans acquired during the treatment course, as defined by existing clinical protocols (see Figure 2), the NA-MIC Kit will provide image registration and segmentation. Registration and segmentation results will be transferred back to the TPS for beam definition and dose calculations. We will then use NA-MIC visualization tools to present these results to the experts, who will review them for clinical validity. A successful adaptive planning workflow will create plans that experts consider more clinically beneficial than the original plans.
Aim 2. We will work closely with Core 1 to explore and optimize deformable registration and automatic segmentation algorithms available in NA-MIC Kit. The most promising algorithms for registration include demon’s, B-spline, and thin-plate splines. For segmentation good candidates include graph-cut and level set methods. Because expert segmentation is available from the original scan, we will design new algorithms that use expert segmentation to guide the segmentation of subsequent scans. Registration and segmentation accuracy will be evaluated by comparison with expert markup. The use of non-rigid registration algorithms for automatic segmentation of images in adaptive radiotherapy, and for mapping of the prior radiation plans onto an updated scan, represent a significant innovation in the fi eld of radiation therapy.
Aim 3. To evaluate adaptive radiotherapy against traditional radiotherapy, we will apply dose response models to hypothetical treatments using both strategies. Plans will be generated based on the initial CT, and simulated on subsequent CT scans. Dose to the clinical target volume (CTV) and organs at risk will be analyzed using site-specific metrics, including minimum dose, maximum dose, and dose volume histograms. The focus of the study will be on proton-beam treatments, but also we will evaluate intensity-modulated radiation therapy (IMRT) for photon treatments. Dosimetry results from this study will be used to predict tumor control probability (TCP) and normal tissue complication probability (NTCP) using logistic and Lyman models [5-7].
Analysis of TCP and NTCP will be used to estimate the percentage of patients who will benefit from the adaptive treatment strategy. The PI, Gregory Sharp, PhD, is a clinical medical physicist with research interests in image-guided radiotherapy. Annie Chan, MD, will lead the clinical evaluation. Dr. Chan is a radiation oncologist who specializes in head and neck cancer, with research interests in proton-beam therapy.
Connections between this work and any of the other 3 proposed DBPs
This DBP shares the basic needs of the other DBPs for new image registration in the presence of pathology, advanced segmentation tools for subject-specific analysis, shape analysis and deformable shape segmentation tools, and longitudinal analysis for effi cient processing of baseline and follow-up scans. The development of new tools and workflows will involve close interaction with Core 1 algorithms and engineering.
Deliverables, Timeline, Impact
The scientific deliverables of this DBP are: (1) to demonstrate the use of an open-source software platform for adaptive radiotherapy, and (2) to evaluate the therapeutic gain of adaptive radiotherapy in protonbeam treatments of the paranasal sinus and nasopharynx. In addition to presenting and publishing our scientific results, we will host two educational workshops dedicated to the use of NA-MIC tools for adaptive radiotherapy.
In year 2, we will host a special 1-day workshop on the MGH campus. During year 3, we will host a tutorial session at a major national or international radiotherapy conference, such as the AAPM Annual Conference or the Annual ASTRO Meeting. MGH investigators will interact directly with NA-MIC Computer Science Core PIs in developing new and refining existing algorithms for addressing the issues inherent to adaptive radiotherapy. DBP activities at MGH will occur in consultation with the Primary Technical NA-MIC DBP Contact Algorithm (Golland) and Engineering (Pieper) contacts. Once completed and rigorously validated, these workflows will (1) be applied to the patient data described above, (2) made available for open dissemination via the NA-MIC website, and (3) form the basis for training and educational materials for NA-MIC investigators and the adaptive radiotherapy community. Results will be featured in presentations at scientific conferences, organized training events/workshops, etc., as a way to disseminate tool capabilities and, where possible, tutorials on how to use the NA-MIC technology for other projects related to adaptive radiotherapy (see also Training and Dissemination Cores). Finally, the DBP PI will attend each NA-MIC All-Hands-Meeting to discuss the DBP with NA-MIC PIs, report on developments, and progress. NA-MIC will benefit from this DBP by exposure to the field of radiotherapy and, in particular, to therapy planning, a clinical area which is known to critically rely on efficient and robust 3D image registration and segmentation.
The specific aims of this project are appropriate in scope for a three-year DBP project. However, we see an opportunity for collaboration on related topics, including adaptive therapy for lung cancer, 4D treatment planning, functional imaging for radiotherapy planning, and workflow management. Following the DBP, we will pursue one or more of these ideas as a collaboration grant with NA-MIC.
References
- Seer 2007. Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics (Bethesda, MD: National Cancer Institute, SEER Program, 2007) 2007.
- Hansen EK, Bucci MK, Quivey JM, Weinberg V, Xia P. Repeat CT imaging and replanning during the course of IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;64(2):355-62. PMID: 16256277.
- Wu Q, Chi Y, Chen PY, Krauss DJ, Yan D, Martinez A. Adaptive replanning strategies accounting for shrinkage in head and neck IMRT. Int J Radiat Oncol Biol Phys. 2009;75(3):924-32. PMID: 19801104.
- Salama JK, Haddad RI, Kies MS, Busse PM, Dong L, Brizel DM, et al. Clinical practice guidance for radiotherapy planning after induction chemotherapy in locoregionally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009;75(3):725-33. PMID: 19362781.
- Burman C, Kutcher GJ, Emami B, Goitein M. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys. 1991; 21:129-35. PMCID: PMID: 2032883.
- Okunieff P, Morgan D, Niemierko A, Suit HD. Radiation dose-response of human tumors. Int J Radiat Oncol Biol Phys. 1995;32(4):1227-37. PMID: 7607946.
- Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45(3):577-87.