Difference between revisions of "2010 Winter Project Week Musco Skeletal Segmentation"
| (23 intermediate revisions by 2 users not shown) | |||
| Line 5: | Line 5: | ||
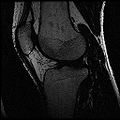
Image:IdealSpgrWater.jpg|Ideal Spgr Water MR image | Image:IdealSpgrWater.jpg|Ideal Spgr Water MR image | ||
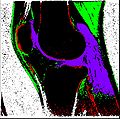
Image:SegmentationOutput.jpg | Segmentation Output | Image:SegmentationOutput.jpg | Segmentation Output | ||
| − | |||
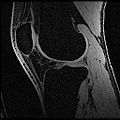
Image:Bones.jpg | Bones | Image:Bones.jpg | Bones | ||
Image:cartilage.jpg | Cartilage | Image:cartilage.jpg | Cartilage | ||
| − | |||
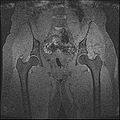
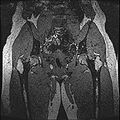
Image:IdealSpgrWaterHip.jpg | SpgrWater_Hip | Image:IdealSpgrWaterHip.jpg | SpgrWater_Hip | ||
Image:IdealSpgrOpHip.jpg | SpgrFat_Hip | Image:IdealSpgrOpHip.jpg | SpgrFat_Hip | ||
| + | Image:HipOut1.jpg | Hip Output | ||
</gallery> | </gallery> | ||
| Line 16: | Line 15: | ||
==Key Investigators== | ==Key Investigators== | ||
* Stanford: Harish Doddi, Saikat Pal, Scott Delp | * Stanford: Harish Doddi, Saikat Pal, Scott Delp | ||
| − | * Kitware: Luis Ibanez | + | * Kitware: Luis Ibanez, Harvey Cline |
| + | * GE Research: Xiaodong Tao | ||
<div style="margin: 20px;"> | <div style="margin: 20px;"> | ||
| Line 22: | Line 22: | ||
<h3>Objective</h3> | <h3>Objective</h3> | ||
| − | The aim of this project is to develop | + | The aim of this project is to develop a methodology for rapid segmentation of knee structures from magnetic resonance (MR) images for subject-specific modeling. The overall goal can be broken down into two specific objectives - |
| + | <BR> | ||
| + | 1. Rapid segmentation of target structures into label maps. | ||
| + | <BR> | ||
| + | 2. Generation of simulation-ready models from existing atlas and label maps of individual structures. | ||
</div> | </div> | ||
| Line 28: | Line 32: | ||
<h3>Approach, Plan</h3> | <h3>Approach, Plan</h3> | ||
| − | + | <i><u>Approach:</u></i> | |
| + | <BR> | ||
| + | Objective 1: We have adopted a multi-contrast MR methodology to segment knee bones and cartilage structures. The algorithm utilizes tissue intensity information from multiple MR contrasts to segment structures of interest. Inputs to the algorithm included n registered MR image sets. The algorithm created an n-dimensional space of voxel intensities associated with the n image sets. The user assigned seed points to the structures of interest, and the algorithm created a cluster center for each structure of interest. Cluster radii were calculated based on standard deviations obtained from seed points, and tissue structures were classified as label maps. | ||
| − | + | For more details, please visit http://www.na-mic.org/Wiki/index.php/Stanford_Simbios_group | |
| + | <BR> | ||
| + | <i><u>Plan: </u></i> | ||
| + | <BR> | ||
| + | a. Incorporate a method to refine label maps (remove undesired bridge connections). | ||
| + | <BR> | ||
| + | b. Implement an algorithm to assign fiducial points to region boundaries for manual adjustments of geometries. | ||
</div> | </div> | ||
| Line 37: | Line 49: | ||
<h3>Progress</h3> | <h3>Progress</h3> | ||
| − | + | * Implemented algorithm to segment structures of interest from multi-constrast MR images. | |
| + | * Generated label maps of knee and hip structures. | ||
| + | * Implemented a pipeline to rapidly refine label maps to isolate regions of interest. | ||
| + | * Identified an approach to trace geometry boundary and automatically assign fiducial points. | ||
| + | |||
</div> | </div> | ||
</div> | </div> | ||
Latest revision as of 16:28, 7 January 2010
Home < 2010 Winter Project Week Musco Skeletal Segmentation
Key Investigators
- Stanford: Harish Doddi, Saikat Pal, Scott Delp
- Kitware: Luis Ibanez, Harvey Cline
- GE Research: Xiaodong Tao
Objective
The aim of this project is to develop a methodology for rapid segmentation of knee structures from magnetic resonance (MR) images for subject-specific modeling. The overall goal can be broken down into two specific objectives -
1. Rapid segmentation of target structures into label maps.
2. Generation of simulation-ready models from existing atlas and label maps of individual structures.
Approach, Plan
Approach:
Objective 1: We have adopted a multi-contrast MR methodology to segment knee bones and cartilage structures. The algorithm utilizes tissue intensity information from multiple MR contrasts to segment structures of interest. Inputs to the algorithm included n registered MR image sets. The algorithm created an n-dimensional space of voxel intensities associated with the n image sets. The user assigned seed points to the structures of interest, and the algorithm created a cluster center for each structure of interest. Cluster radii were calculated based on standard deviations obtained from seed points, and tissue structures were classified as label maps.
For more details, please visit http://www.na-mic.org/Wiki/index.php/Stanford_Simbios_group
Plan:
a. Incorporate a method to refine label maps (remove undesired bridge connections).
b. Implement an algorithm to assign fiducial points to region boundaries for manual adjustments of geometries.
Progress
- Implemented algorithm to segment structures of interest from multi-constrast MR images.
- Generated label maps of knee and hip structures.
- Implemented a pipeline to rapidly refine label maps to isolate regions of interest.
- Identified an approach to trace geometry boundary and automatically assign fiducial points.